Crinecerfont can improve CAH
What is CAH and its cause?
In response to stress, the body releases CRH (corticotropin-releasing hormone) from the hypothalamus, which signals the pituitary gland via CRF1 (corticotropin-releasing hormone receptor 1) to produce ACTH (adrenocorticotropic hormone). ACTH further stimulates the adrenal gland present over the kidney to synthesize cortisol, aldosterone, and androgens required for normal metabolic and sexual development. Cortisol is the stress hormone, aldosterone regulates blood salt balance, and androgens are sex hormones. Cortisol in turn regulates its level by inhibiting ACTH and CRH secretion. The defect in this feedback inhibition leads to CAH (congenital adrenal hyperplasia). It is caused by a deficiency of the 21 hydroxylase (CYP21 gene) enzyme1, which synthesizes cortisol and aldosterone in the adrenal glands. The absence of cortisol stimulates ACTH from the pituitary, which keeps increasing the sex hormone production but no cortisol or aldosterone (Figure 1). CAH is a genetic disorder and is transferred in an autosomal recessive pattern. There are two kinds of CAH- classical and non-classical. Non-classical CAH is a milder form, where cortisol is present at a lower level than in healthy individual. Its symptoms appear either in adolescence or adulthood, sometimes never. The classical CAH occurs in 1 in 15000 and the non-classical form in 1 in 1000 births worldwide2.
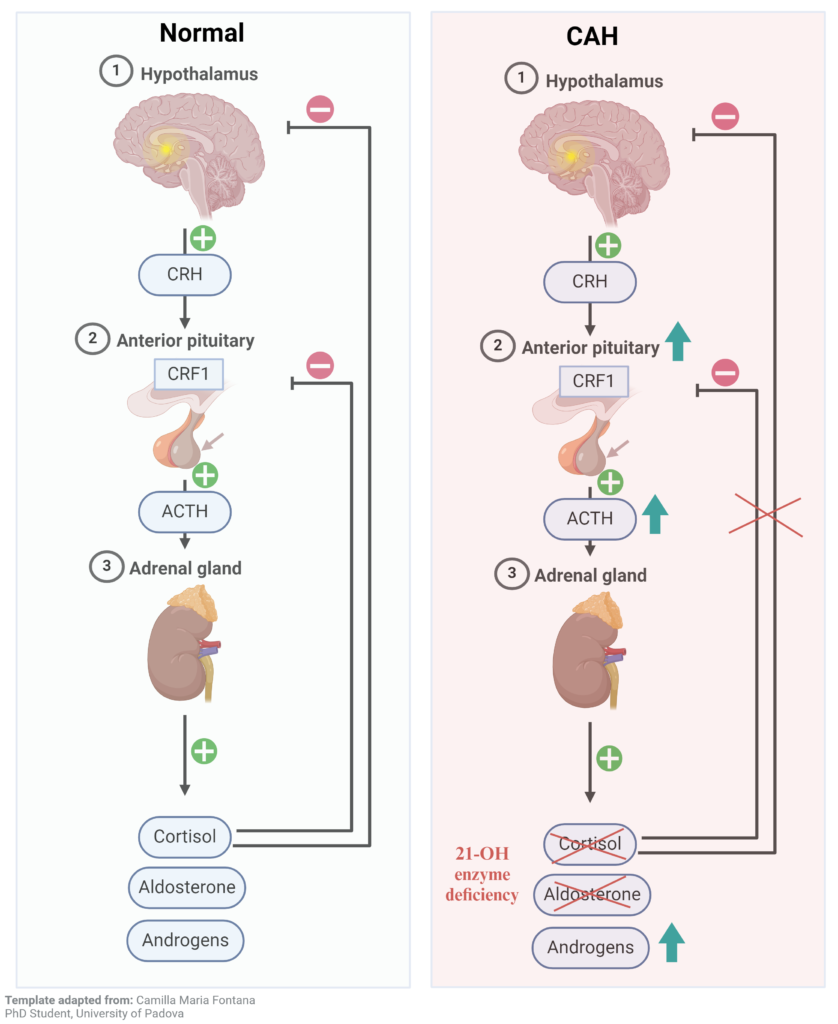
Figure 1 – Congenital adrenal hyperplasia (CAH) cause
Symptoms3
Classical CAH is life-threatening if not found early and treated. It can lead to shock and coma. Most of the symptoms of classical CAH are due to low aldosterone and high sex hormones. Low aldosterone causes loss of sodium in the urine and the body cannot maintain blood pressure, leading to dehydration, irregular heartbeat, vomiting, diarrhea, and shock. Excess sex hormones cause sexual development disorders, the genitalia look abnormal and do not represent either male or female sex organs. Other symptoms include premature puberty, abnormal menses in females, non-cancerous testicular tumors in males, and infertility.
Nonclassic CAHmay be asymptomatic. If not, the symptoms are mild such as signs of early puberty, acne, infertility, etc.
Treatment and its challenges
In classic CAH, the treatment starts right after birth and continues throughout life. Treatment includes drugs that supplement the missing hormones (glucocorticoids). A few examples of glucocorticoid drugs are hydrocortisone for children; prednisone, dexamethasone for adults to supplement cortisol, and fludrocortisone for aldosterone. Many glucocorticoids are available from short to long acting depending on the need. In addition to drugs, surgery is also required to correct the baby’s genitals4. The need for these hormones in the body fluctuates during different conditions such as stress. Accordingly, doses of these drugs need to be adjusted. Excess cortisol can lead to cushion syndrome—which can cause weight gain, high blood pressure, glucose, bone loss, etc. Too much or too little of any hormone is bad, thus care must be given when taking supplements.
Due to varying levels of cortisol in the body, a high dose of glucocorticoids is required to maintain the physiological levels. These high doses can in turn lead to reduced growth, increased obesity, hypertension, and insulin resistance5. Many efforts are being directed to reduce glucocorticoid dose while improving CAH. One such class of drug is CRF1 antagonist which can reduce ACTH secretion and excess sex hormones production.
Crinecerfont can improves CAH
Crinecerfont is a CRF1 antagonist that reduces glucocorticoid doses and still improves CAH. A randomized double-blind phase 3 clinical trial of crinecerfont was conducted by Neurocrine Biosciences in both children and adults. They tested its efficacy by measuring the reduction in levels of sex hormones – 17 hydroxyprogesterone and androstenedione – and glucocorticoid dose. The findings of the study are summarized in Figure 2. Crinecerfont reduced the dose of glucocorticoid by 23.5% and 17% in children and adults respectively while reducing the sex hormone levels too. The details of the study can be found here for children, and adults.
Currently, crinecerfont is under the process of priority review with the FDA, and its decision is expected by the end of 20246.
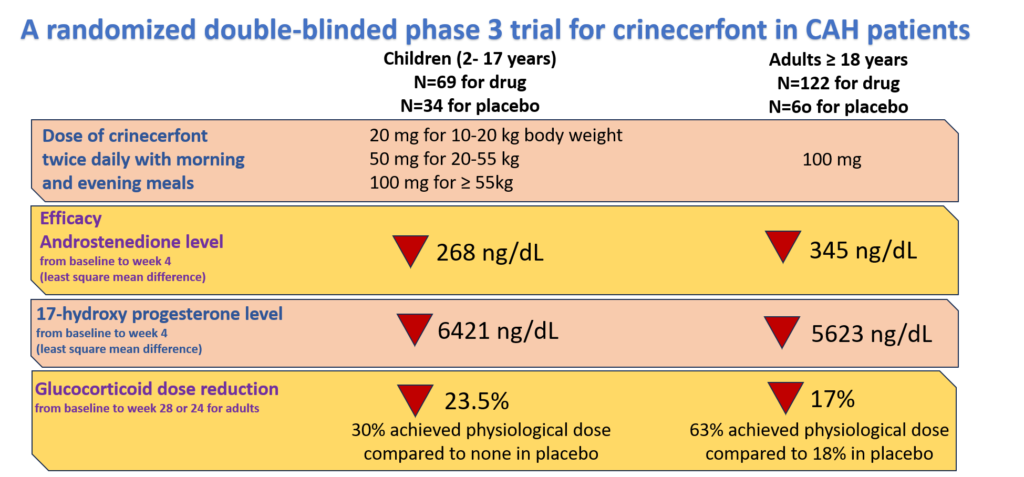
Figure 2. Phase 3 clinical trial of crinecerfont in children and adults.
Resources:
1. Parsa AA, New MI. Steroid 21-hydroxylase deficiency in congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. Jan 2017;165(Pt A):2-11. doi:10.1016/j.jsbmb.2016.06.015
2. Merke D, Kabbani M. Congenital adrenal hyperplasia: epidemiology, management and practical drug treatment. Paediatr Drugs. 2001;3(8):599-611. doi:10.2165/00128072-200103080-00005
3. Congenital Adrenal Hyperplasia. Accessed September 6, 2024, https://my.clevelandclinic.org/health/diseases/17817-congenital-adrenal-hyperplasia
4. Treatments for congenital adrenal hyperplasia. Accessed September 6, 2024, https://www.nichd.nih.gov/health/topics/cah/conditioninfo/treatments
5. Mallappa A, Merke DP. Management challenges and therapeutic advances in congenital adrenal hyperplasia. Nat Rev Endocrinol. Jun 2022;18(6):337-352. doi:10.1038/s41574-022-00655-w
6. Neurocrine Biosciences https://neurocrine.gcs-web.com/news-releases/news-release-details/neurocrine-biosciences-announces-us-fda-accepts-new-drug-0


