MASH (Metabolic dysfunction associated steatohepatitis) is a severe form of non-alcoholic liver disease, previously known as NASH (Non-alcoholic steatohepatitis). It is caused by increased fat deposits and inflammation in the liver despite low alcohol consumption. This further leads to liver scarring called cirrhosis. Untreated cirrhosis causes irreversible damage to the liver leading to its failure and cancer. MASH affects 2-5% of Americans and is strongly associated with diabetes and obesity1,2.
Symptoms
The symptoms of MASH include abdominal swelling, itchy skin, shortness of breath, swelling of legs, jaundice, etc. It is diagnosed by pathological examination of liver biopsy. The life expectance after early diagnosis of MASH is 9 to 12 years. If diagnosed late once cirrhosis has progressed, the life expectancy is only 2-5 years without a liver transplant3. The disease progression in turn depends on the metabolic health of patients. Thus, MASH patients are recommended to eat healthy food and exercise regularly.
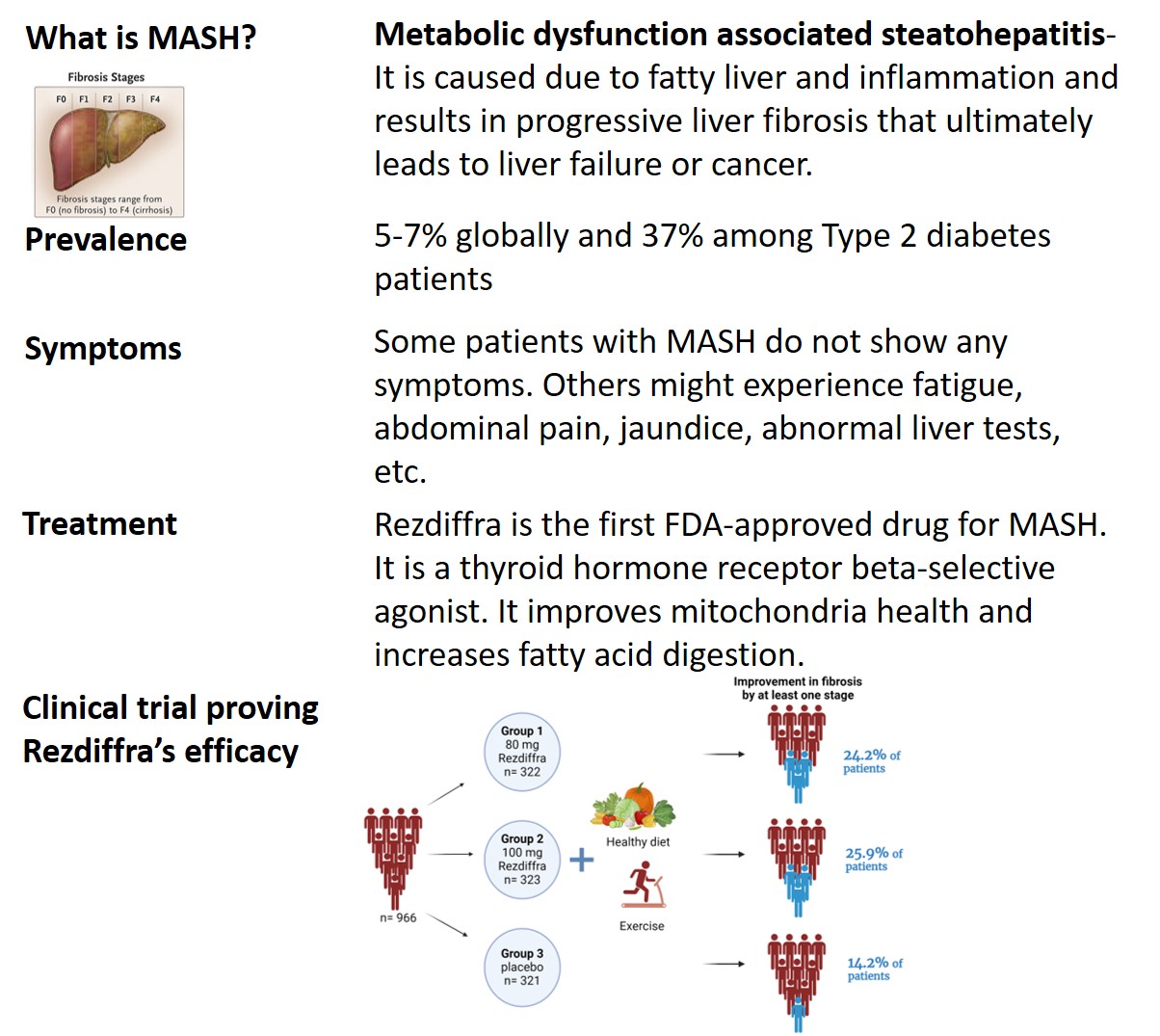
Treatment
There is no cure for MASH, the FDA allowed accelerated approval for drugs that can improve or at least reduce its progression. On March 14, 2024, the FDA approved Rezdiffra for patients with MASH4 along with exercise and a healthy diet. Rezdiffra (resmetirom) stimulates thyroid hormone receptor beta (THR-β). Increasing THR-β further improves mitochondria function including fat digestion, and reduces fibrosis. In this clinical trial, patients with MASH were divided randomly into 80 mg, 100 mg, and placebo groups, with about 320 patients in each group. The drug was given every day once orally for 52 weeks and liver biopsy samples were compared before and after the drug. During the study duration, patients also received nutrition and exercise counseling. At week 52, improvement in fibrosis was observed in 24.2% of people in the 80 mg group and 25.9% in the 100 mg group as compared to 14.2% in the placebo. The most common side effects observed were diarrhea and nausea. These results encouraged using Rezdiffra to improve fibrosis, thus slowing MASH progression. However, most of the patients in the trial were white; thus, it needs to be tested in other ethnic groups too. Further details on the study can be found here.
Who makes Rezdiffra
Rezdiffra is a product of Madrigel Pharmaceutical. It is a small biopharmaceutical public company of about 300 employees and is mostly focused on NASH. A yearly supply of Rezdiffra costs about $47,400 without insurance.
Resources
1. Hamid O, Eltelbany A, Mohammed A, Alsabbagh Alchirazi K, Trakroo S, Asaad I. The epidemiology of non-alcoholic steatohepatitis (NASH) in the United States between 2010-2020: a population-based study. Ann Hepatol. Sep-Oct 2022;27(5):100727. doi:10.1016/j.aohep.2022.100727
2. Younossi ZM, Henry L. Understanding the Burden of Nonalcoholic Fatty Liver Disease: Time for Action. Diabetes Spectr. Winter 2024;37(1):9-19. doi:10.2337/dsi23-0010
3. Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. May 20 2016;17(5)doi:10.3390/ijms17050774
4. Harrison SA, Bedossa P, Guy CD, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med. Feb 8 2024;390(6):497-509. doi:10.1056/NEJMoa2309000


